New Guidance for Industry- Acceptable Intake Limits for NDSRIs and a New Webpage from FDA
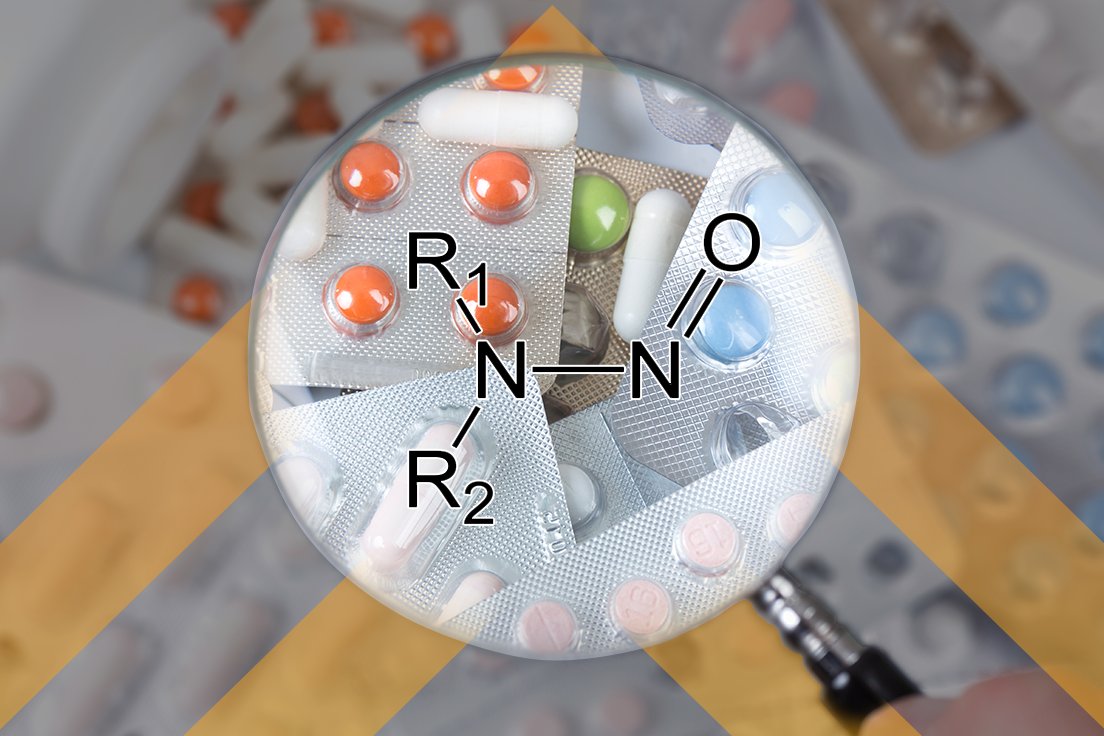
Unless you’ve been living under a (pharmaceutical) rock, you are aware of the struggle that both FDA and industry are having with Nitrosamines impurities in pharmaceutical products. As a reminder, APIs are at risk of forming nitrosamine drug substance-related impurities (NDSRIs) when they contain secondary amines or dimethyl tertiary amines. We have published many blogs […]