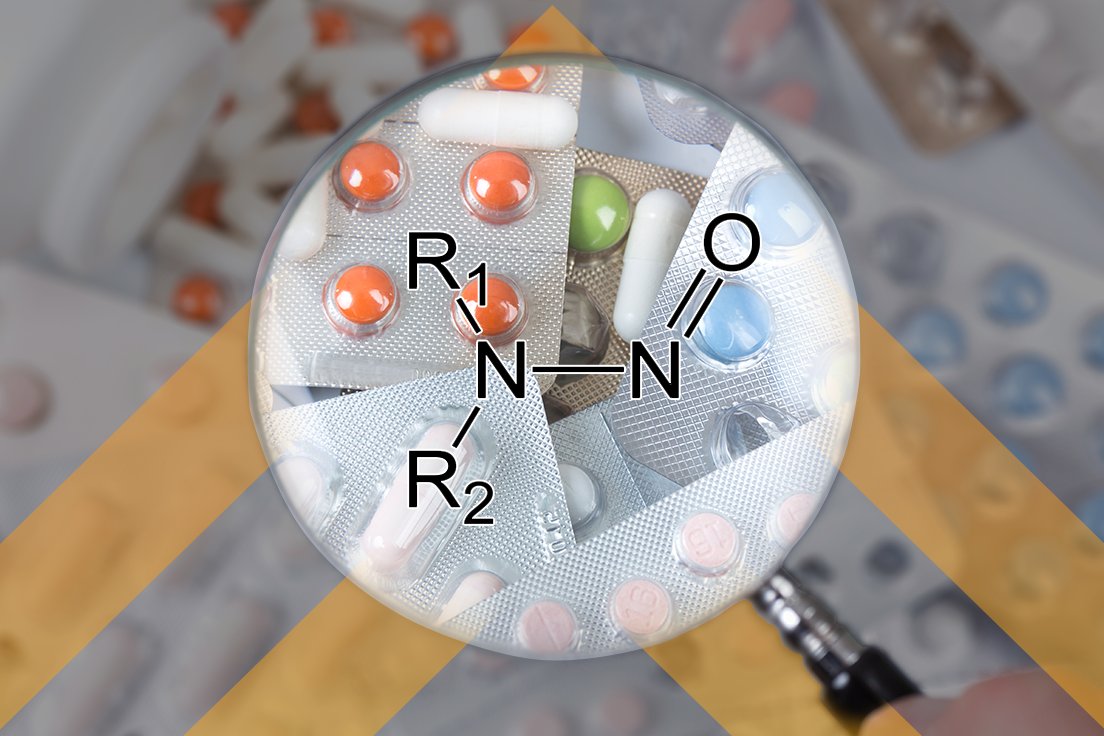
Two years after the FDA guidance on Control of Nitrosamines Impurities in Human Drugs and nearly a year after the publication of USP’s General Chapter <1469> Nitrosamine Impurities, where are we now and what have we learned? That was a question posed recently at the Association of Accessible Medicine’s GRx+Biosims 2022 conference. The session devoted to nitrosamines contained several presentations from the FDA and industry as well as a representative from USP. This breakout session was well attended, demonstrating the continued interest in this critical topic, and the Q&A session went overtime as no one wanted to cut the time short and join the networking reception (which is really saying something for a conference that’s back in person for the first time in three years, full of colleagues and friends)!
The speakers did an excellent job of providing background information on simple nitrosamines and the more complex nitrosamine drug‑substance‑related impurities (NDSRIs) and explaining the differences between the two categories, as well as why the latter category is causing so much trouble for everyone.
Dr. Robert Heflich of the FDA discussed the difficulty in using the Ames test to evaluate mutagenicity for NDSRIs as they can produce negative results in this test while still being known mutagens. His lab at the National Center for Toxicological Research is evaluating some variations in the testing protocol regarding identifying sensitive cell strains and defining optimized testing conditions to generate positive results for known mutagenic NDSRIs. Dr. Andre Raw of the OPQ’s Office of Lifecycle Products provided a good overview of the FDA guidance and the possible mitigation strategies that can be employed by manufacturers to reduce NDSRI levels in generic products, including evaluating nitrite levels in ingredient sources and potential ways to reformulate to prevent nitrosamine formation. Dr. Edmond Biba provided an overview of the activities being undertaken by USP, including the history of the development of General Chapter <1469> and its ongoing efforts to create reference standards and raise awareness of the issue through training and outreach (including videos on YouTube). Dr. Martin Ehlert of Apotex provided some perspective on the prevalence of nitrosamines and the impact on patients that highlighted entire therapeutic classes (such as beta blockers and ACE inhibitors) that now have a confirmed risk of complex nitrosamine impurities. It is estimated that about 20% of current small molecule generic drugs have a secondary amine functional group which, therefore, poses a risk of NDSRI formation. Dr. Raphael Nudelman of Teva Pharmaceuticals provided an overview of an approach that was recently proposed to EMA that would allow the use of a “read‑cross” approach to set interim limits while additional data is gathered to verify the risk and determine the specific impurity control limits for NDSRIs and better define acceptable intake (AI) limits. He also further highlighted the issues with the Ames test and the in vivo comet and transgenic rodent (TGR) gene mutation assays that are difficult to execute, with few CROs having the capability to perform TGR and none having any capacity until well into 2024, therefore reinforcing the need for an interim approach like the one proposed to EMA.
The Q&A was lively, with several key points being when the maximum daily dose of a product causes issues setting analytical targets – which is where the “read‑across” approach to setting interim limits was proposed to offer a solution to setting interim targets to allow method development and qualification for gathering data while the NDSRI was further evaluated. Several questions focused on Q1/Q2 in relation to ANDAs and their RLD when divergent approaches were taken by each manufacturer and when BE testing might be triggered. Reformulation issues were the subject of many questions, where inactive ingredient database (IID) limits were questioned in relation to materials that worked for the reformulation but were not present in the database, and whether reformulation supplements would be granted priority review. Spoiler alert: It didn’t sound as though the FDA was willing to allow ingredients that were not listed in the IID at the same levels to be included in ANDA products and it would not grant priority review unless there was a shortage situation for the product. All good information, but the key takeaway at the end of the day was that there should be one singular focus – the safety of the patient. It is up to ANDA holders to provide a well‑reasoned and science‑based justification for their proposed limits and mitigation strategies, if necessary, but industry is also asking that the FDA be a little more flexible and allow the use of interim, science‑based limits to support interim limits upon which to base initial assays while the mutagenicity data is collected to verify and confirm final AI limits.
Life is all about give and take, and we all want to support the safe use of generic medicines by the American public, so let’s work together on finding a pathway and walk it together. Please keep your eyes open for our soon-to-be available White Paper on this issue. If you have any questions about risk assessment for nitrosamine formation in your firms’ products, please reach out to Lachman Consultants at LCS@LachmanConsultants.com.