APPROVAL AND TENTATIVE APPROVAL ACTIONS NUMBER FOR ANDAS JULY 2020
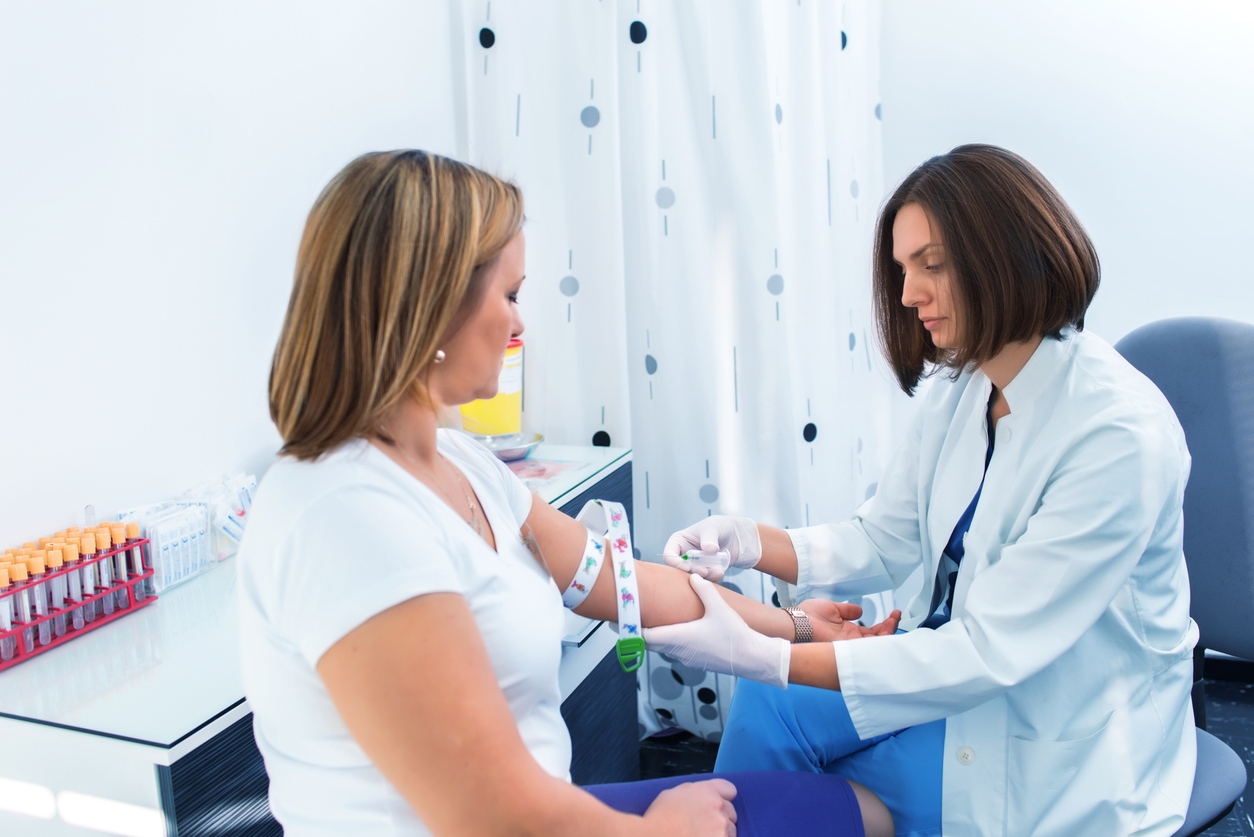
As of August 5, 2020, the FDA had posted 69 full approval actions and 9 tentative approval actions for ANDAs for July 2020. The total of 78 approval actions for the month of July fall short of the 87 Approval actions in June but appears to be consistent with the last four month’s totals. Some […]