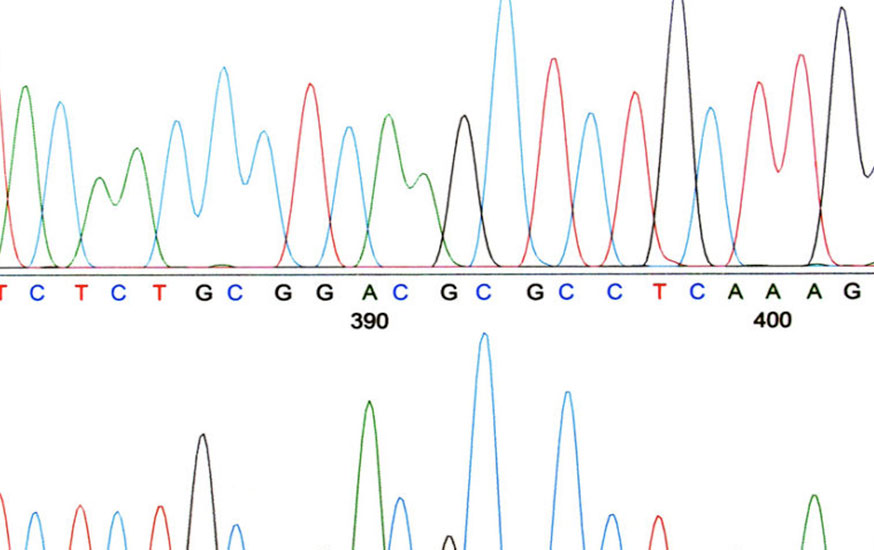
Are All Methods Equivalent?
The use of alternate methods, especially for USP methods, some of which are rather old, has long been a question that both the industry and the FDA have contemplated. Alternate methods can be easier to use and, in some instances, more accurate and reliable. But how can you demonstrate that an alternate method is indeed […]