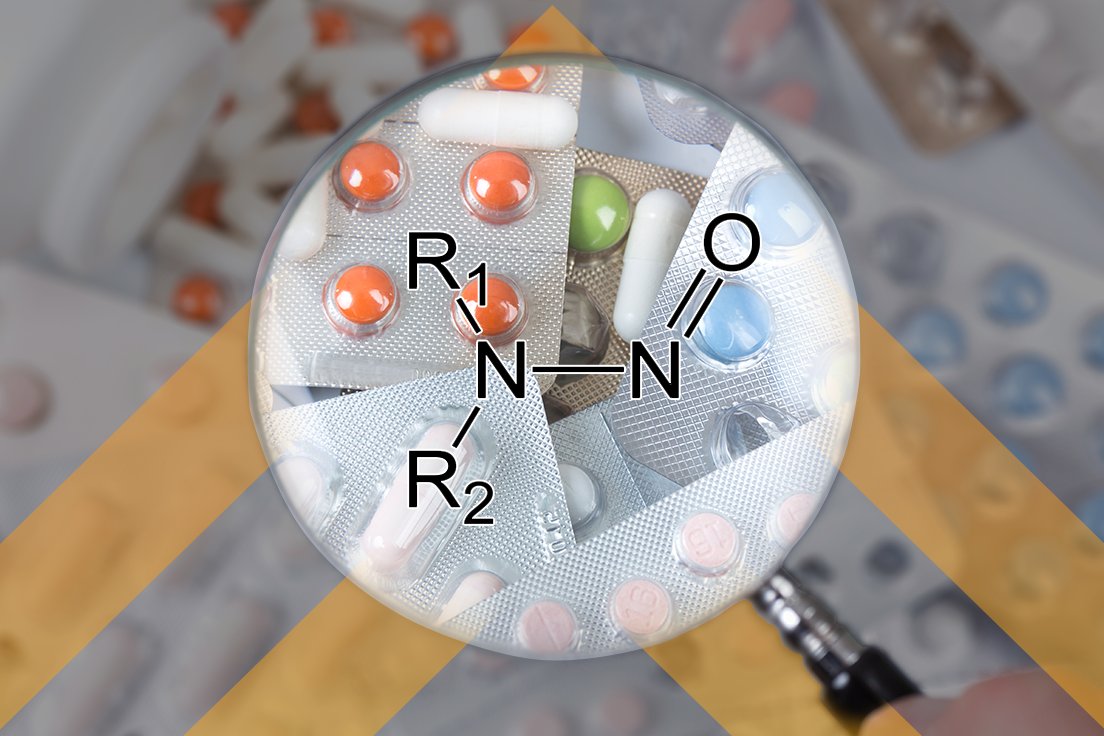
What is Going on With Nitrosamines?
Do you have an ANDA, supplement, or controlled correspondence in at FDA awaiting resolution of a nitrosamine impurity issue? Has it been months overdue? You are not alone. OGD has been silent on those submissions as they navigate the widely expanding nitrosamine issue. My first thought is what happened since 1984, when Hatch-Waxman was first […]