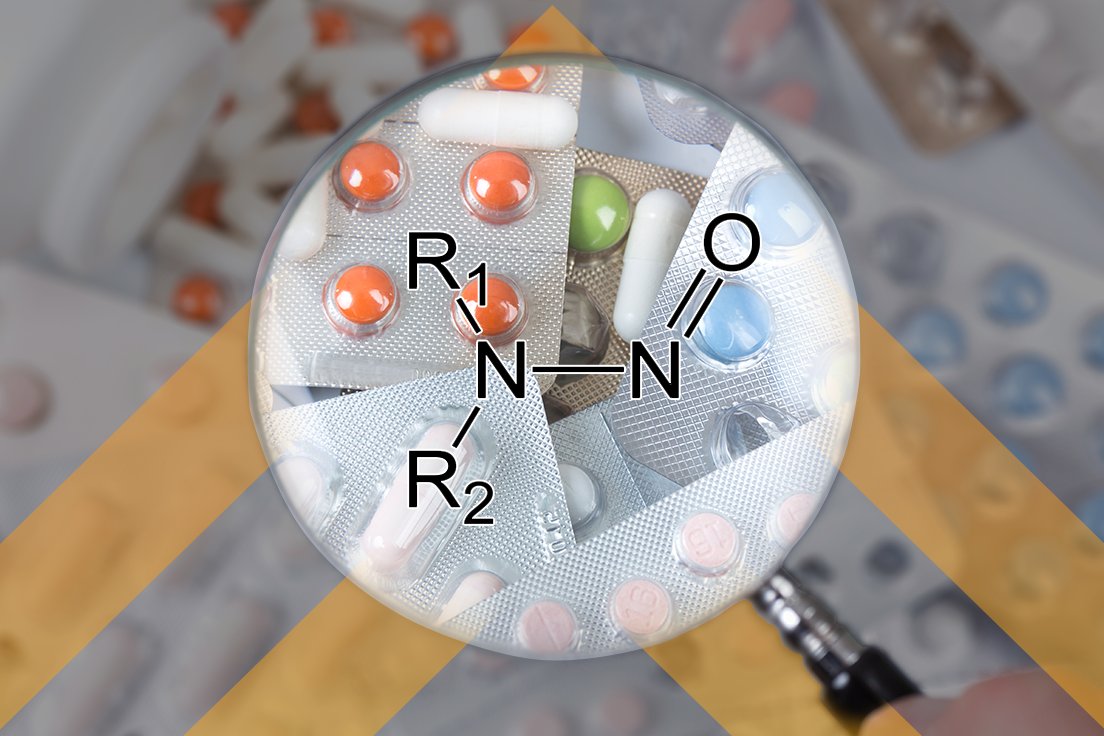
Do you have an ANDA, supplement, or controlled correspondence in at FDA awaiting resolution of a nitrosamine impurity issue? Has it been months overdue? You are not alone. OGD has been silent on those submissions as they navigate the widely expanding nitrosamine issue. My first thought is what happened since 1984, when Hatch-Waxman was first approved and why did it take almost 30 some years for the nitrosamine problem to reach the front burner? Is it related to increased and improved analytical methods? Was there always a problem that was hidden because techniques were not available to uncover these impurities? I sure as heck don’t know the answer but I do know that FDA is working diligently on solving the problem. But clearly not fast enough for industry.
In yesterday’s pre-publication of the Federal Register we found a Notice entitled, Identification, Assessment, and Control of Nitrosamine Drug Substance-Related Impurities in Human Drug Products (here) “announcing the establishment of a docket to solicit public comments on the identification, assessment, and control of N-nitrosamine (nitrosamine) drug substance-related impurities (NDSRIs) that may be considered by the Agency in its regulation of these types of impurities in drug products.”
The document reports on what the FDA knows to-date and provides some recommended solutions to potentially mitigate the problem, but it seems clear that the Agency is also struggling to provide the best information to resolve this problem. In the Notice, FDA describes its problem with confidential information that it may receive from other applicants (whether NDA or ANDA holders) and its problem with sharing that information publicly. In my opinion, the Notice clearly implies that the FDA is asking for industry’s help in solving this problem by requesting that the industry collaboratively and publicly share its research on appropriate animal models and other testing to obtain an appropriate acceptable intake (AI) limit for various nitrosamine impurities and there seems to be new ones found every month.
While this does not bode well for an early resolution to the problem, it certainly (in my opinion) sheds some light on the difficult time and the bind that the FDA may be in. The FDA also notes that it does not want to have multiple parties performing the same duplicative testing on animals as this is counter to the goal of such limited testing. But as in the widely known phrase “What’s a mother to do?”, it appears that FDA might be up against a problem that could delay review and approval of lot of applications. And, as those applications age, FDA will miss more GDUFA dates, the mean and median approval times for these applications will continue to creep up, and medicines will not get to patients in a timely manner. It has almost become a waiting game to see when the next shoe will drop, that is, what will be the next product to succumb to the nitrosamine boogeyman. Stay tuned! We are as anxious to know as you are!