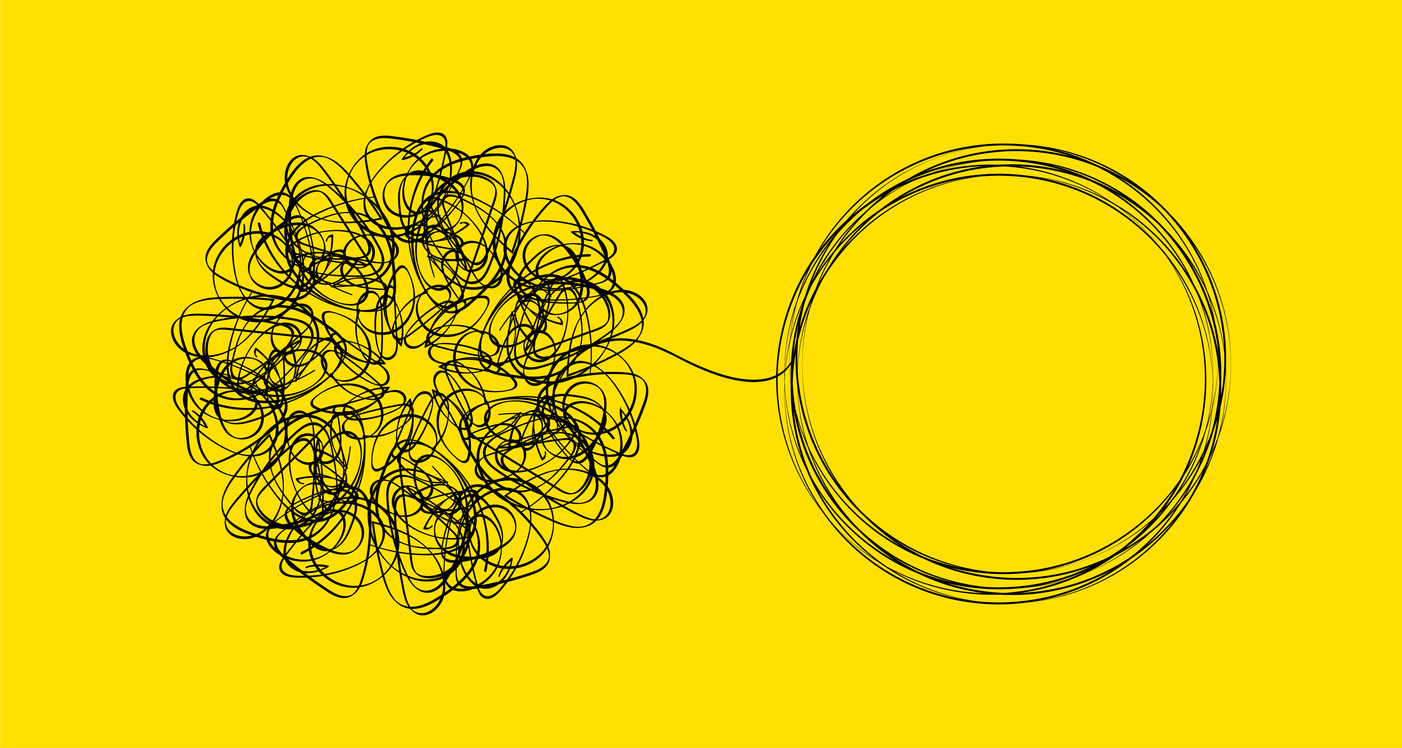
QMM Still a Hot Topic
The FDA Quality Management Maturity (QMM) program has generated an increased interest in quality culture in the pharmaceutical industry. The Center for Drug Evaluation and Research (CDER) has established a program to encourage manufacturers of drugs (including biologics), with the stated purpose of implementing QMM programs to: Foster a strong quality culture mindset; Recognize establishments […]