Hundreds of FDA staff, industry professionals, lawyers, and others gathered in Washington, D.C. on May 17th and 18th to participate in the 2023 Food and Drug Law Institute (FDLI) Annual Conference. The agenda covered a broad range of current regulatory and legal issues facing the food, drug, medical device, biologics, and tobacco industries. Lachman Consultant Services was there to capture some of the highlights.
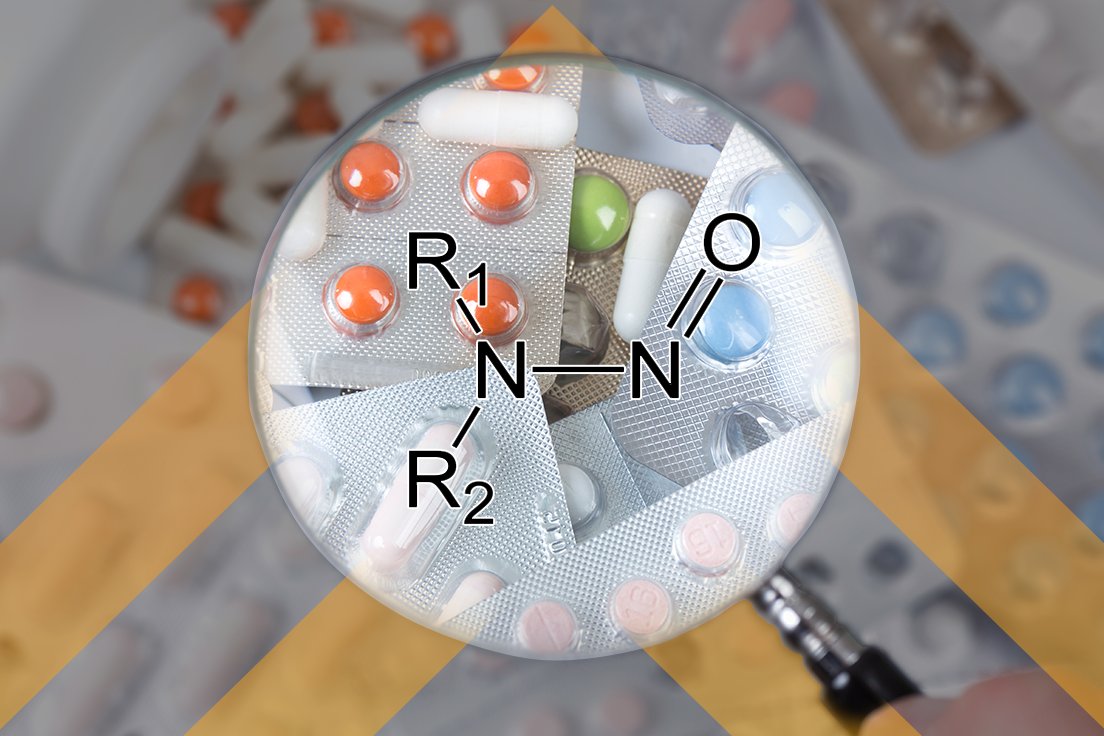
Nitrosamines and other impurities were a topic of keen interest, with FDLI dedicating a full session to the topic, as well as being mentioned as a top priority by CDER’s Director, Patrizia Cavazzoni. Carcinogenic nitrosamine impurities were first detected in certain drug products in 2018, triggering worldwide recalls of important drug products. Since then, global regulators, including the FDA, as well as industry have grappled with mitigation strategies for controlling the levels of these impurities in drug products. In the breakout session dedicated to “Regulating and Mitigating Nitrosamines and Other Impurities in Drug Products,” CDER’s Senior Policy Advisor, Jason Bunting, highlighted some recent updates from the FDA on this evolving issue. In November 2021, the FDA published two examples of mitigating strategies that manufacturers may consider for inhibiting formation of nitrosamine drug substance‑related impurities (NDSRIs) by incorporating antioxidants in the drug product’s formulation and incorporating excipients to produce a neutral or basic environment. Most recently, on May 4, 2023, the FDA issued a notice in the Federal Register (see Lachman’s previous post on this FR notice here) soliciting public comments on the identification, assessment, and control of NDSRIs that may be considered by the Agency in its regulation of these types of impurities in drug products. The FDA is also collaborating with the Health and Environmental Sciences Institute (HESI) to develop an enhanced Ames test to generate mutagenicity data for these impurities and identify research gaps.
Perspectives from outside counsel and industry insiders emphasized the challenges manufacturers are facing in this evolving space, including laboratory capability to detect these impurities and develop appropriate test methods, absence of available safety data to establish acceptable intake limits for NDSRIs, and reliance on sometimes spotty information from suppliers to effectively assess risk from raw materials. All participants agreed on the importance of collaboration between global regulators and industry to produce and share data that will help inform the next decisions and actions for controlling the levels of these impurities to prevent harm. See other Lachman blog posts on the subject of nitrosamines here, here, and here.