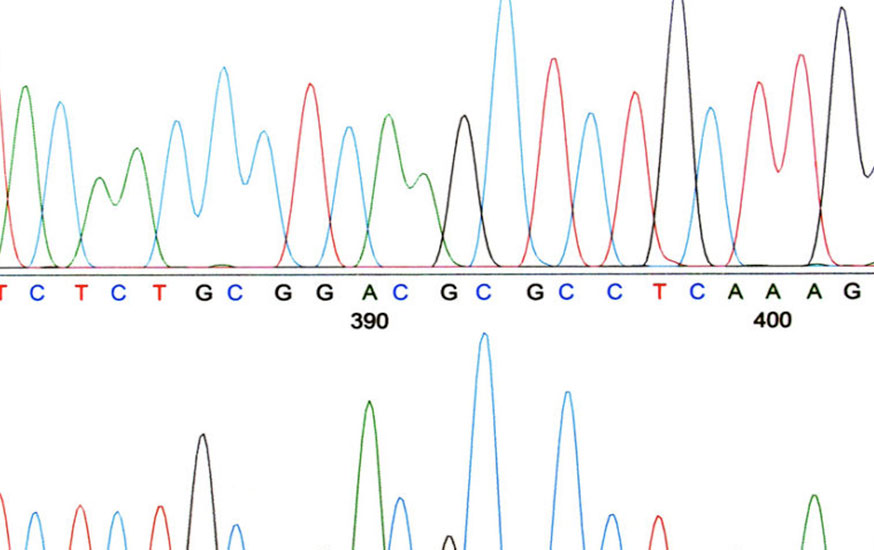
Extraneous Chromatographic Peaks – What’s a Lab to Do?
Recently, the United States Pharmacopeia and National Formulary (USP-NF) announced a three-month public consultation period on a proposal to change the approach to impurity reporting thresholds for drug products and drug substance monographs[i]. This activity is part of the USP-NF’s ongoing efforts to ensure that monographs reflect technical advances and current regulatory expectations. In 2016, […]