During her session yesterday at the AAM Annual Meeting, Iilun Murphy, M.D. provided additional details on the 2023 OGD metrics, indicating that there were 950 ANDAs approved and tentatively approved (782 and 172 approvals, respectively). Of the approvals, there were 90 first generics, along with 83 generics with Competitive Generic Therapy Designation, and 111 complex generics.
Dr. Murphy also noted that one of the new reports required under GDUFA III might not have been as clear as the Agency had wanted and assured the audience that it would look to make the report more transparent and understandable. She was speaking of the GDUFA III Performance Metrics Report, which we reported on and explained in a previous blog post here.
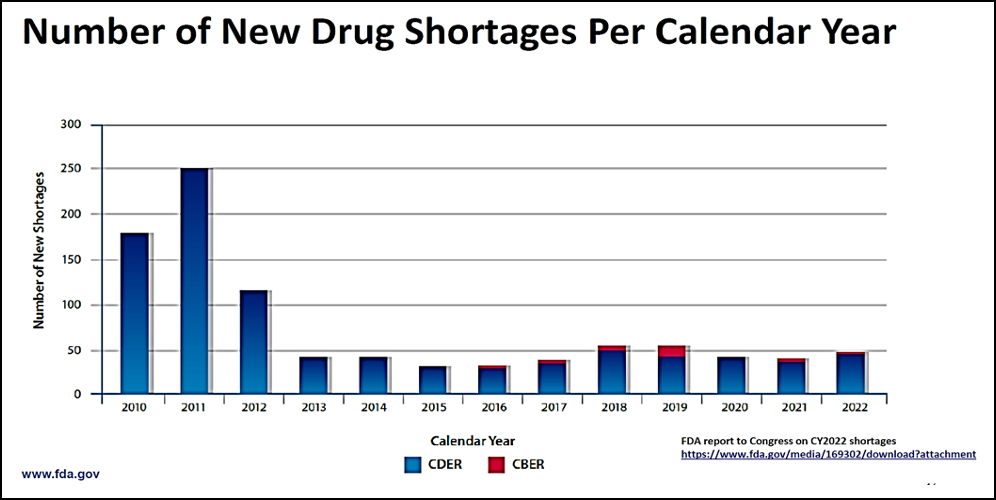
Jacqueline Corrigan-Curay, J.D., M.D., Principal Deputy Center Director of CDER, made another interesting point in her presentation yesterday, outlining the number of national drug shortages that the FDA reported per calendar year (reproduced below) and indicating that the press has reported an ever-increasing number of shortages, but the data belie that assertion. However, she did note that local or regional shortages may far exceed the FDA’s reported numbers, and it may be that these reports are the ones that have drawn additional public attention. However, any shortage, whether national or local, is important because if a patient cannot get their prescription filled, it is clearly a serious issue for the patient.
We will be reporting more from the Annual Meeting over the next few days so keep your eyes on this space for additional updates.



