Marty Shimer, Executive Director at Lachman Consultants, wrote a great post (here) about ANDA suitability petitions and the new GDUFA goal dates being assigned to them. His post gives us hope that the new system in place will provide some meaningful timelines for actions on ANDA suitability petitions used to make certain permissible changes from reference listed drugs (RLD) if those changes do not require additional studies to support the safety or effectiveness of the proposed change. Those changes include only: changes in strength, change in dosage form, change in route of administration (to date no petition has ever been approved for a change in route of administration because it almost always would impact safety or effectiveness), or a change in ingredient in a combination product if one ingredient for another of the same therapeutic category from that of the RLD. OK, now that is the 10-second primer on ANDA suitability petitions.
The reason I bring this up is, when looking at the GDUFA III first 2024 quarterly report of metrics required under FDARA Title VIII Sections 807 and 805 (here) posted today, I noticed something that I had forgotten. One of the reported metrics included in the report is the number of ANDA suitability petitions pending a substantive response from the FDA and the number of petitions pending a substantive response from the Agency that are pending for 180-days or more. The 2024 first quarter report only has an “*” in the columns of results of suitability petitions. Then I looked up what the “*” meant in the chart and found that this metric is only reported once a year. Tough to track progress during the year when looking at something that only is reported once a year!
Let’s take a look at the FY 2023 report below:
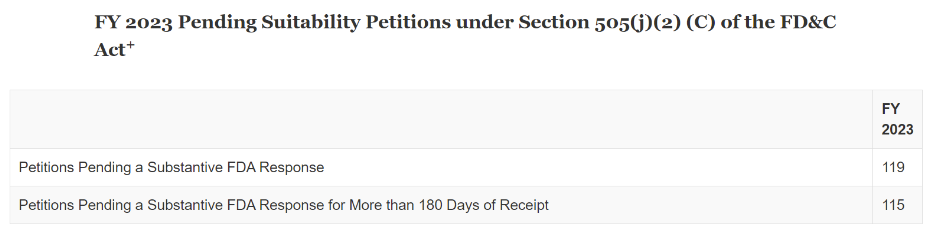
As you can see, there was not much progress made in 2023 in that, of the 119 petitions pending a substantive review, 115 were more than 180 days old. It is also interesting that the FFDCA still requires ANDA suitability petitions to be responded to within 90 days! Clearly that deadline has been thrown out the window as so many of these petitions have been languishing at the Agency for years. Under GDUFA III, OGD will try to meet a goal of 180-days (twice the statutory requirement) only on a certain number of petitions (see the Shimer post above for more details). In addition, if you want an older petition to receive a goal date, OGD suggests withdrawing the old petition and resubmitting it now (after the start of FY 2024, October 1, 2023). Many firms have either withdrawn older petitions and resubmitted to gain a goal date, while others have just withdrawn older petitions because the proposed product is no longer relevant to their portfolio.
Don’t think that I don’t appreciate the work FDA has gone through to revitalize the petition process, because I do. As the first chairman of the ANDA suitability petition committee in 1985, the process is near and dear to my heart. And while many firms have had a petition sitting for several years, now having to wait another 6-months for a resolution is I am sure somewhat discouraging, but at least it is a start. So, thanks OGD for providing the new timelines and goal dates, I can’t wait to see what the annual report will look like at the end of the FY year.



