The Office of Generic Drugs approved eighty-two first-time generic products in FY 2023 for fifty-two different products. For example, the OGD approved sixteen ANDAs on the same day for Lisdexamfetamine Dimesylate Capsules (the generic for Vyvanse) on August 25, 2023. Each of these is counted as a first approval because they were all approved on the same day; however, the total number of different products (when removing first duplicates on the same day) was fifty-two.
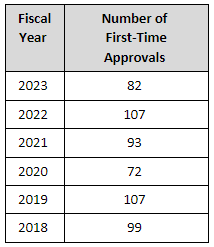
It is difficult to say much about how many first-time generics are approved in relation to previous years because so much depends on patent and exclusivity issues that dictate how and when an ANDA is submitted and when an application can first be approved. So, I won’t make any assumptions and just present the facts. Below is a list of the number of first-time generic approvals for the last six FYs.
To see all the first generic approvals for 2023, please click here.
The OGD continues to prioritize ANDAs and works as best it can to approve generic applications on the first day that they can legally be approved. And (of course), the OGD does the same for other ANDAs where there is no generic competition in the marketplace. Let’s give the OGD a rousing round of applause for all its hard work!



