Biosimilars are advancing the cause of affordable medications; however, in some instances, patient savings do not appear to be the primary concern of Pharmacy Benefit Managers (PBMs). The Association of Accessible Medicines (AAM) points to the fact that “[j]ust two weeks ago, the U.S. biosimilars industry entered a new phase when eight biosimilar versions of Humira launched with prices reaching as low as 85 percent below the brand list price. Humira is the bestselling drug in the U.S., and new biosimilar competition represents a major expansion in patient access to treatment and savings. That said, the benefits to patients won’t materialize overnight.” Part of the reason for this is the sometimes slower uptake of biosimilars by prescribers, but most often the stumbling block revolves around the PBMs and their relationship to the payors and their convoluted formulary systems.
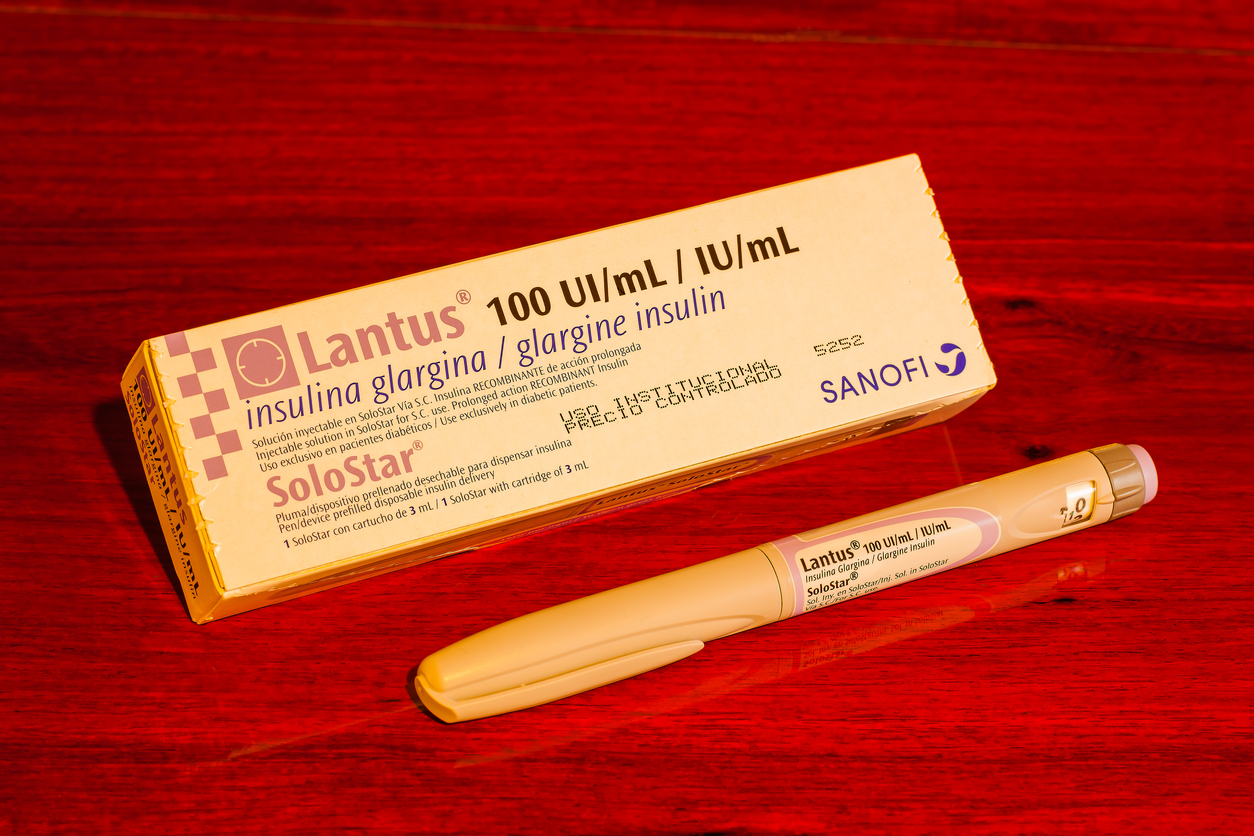
AAM cites, in its post here, the example of the biosimilar insulin “Semglee and its unbranded insulin glargine launched as the first pharmacy-distributed, fully interchangeable biosimilar. This is a biosimilar version of the blockbuster diabetes medication, Lantus, and can be substituted at the pharmacy counter.” In 2021, Semglee launched at a slight discount, but the unbranded version was marked down to 65% of the brand‑name product. AAM further states, “[T]his allows pharmacy benefit managers (PBMs) creating drug formularies to choose between a higher list price that allows them to generate revenue from a high rebate or a significantly lower list price that results in lower costs to patients. Since a lower list price means lower costs for patients, the choice would seem obvious. But an independent analysis last year found that PBMs largely chose the higher-priced version.”
The post describes the results of a study by IQVIA on the insulin market and how the decisions PBMs make may be more in tune with protecting payors rather than patients. The article further notes that “Insulin Glargine accounts for over 50% of new written prescriptions in Medicare, but only 30% of new-to-brand fills due to payer controls.” Sound familiar to what is happening in the small‑molecule generic market? The federal government, and public and private enterprises, are focusing their attention and legislative initiatives on PBMs and how they influence pricing, as well as what patients pay for such products. Read the full post at the link above and see what you think.