First, please be aware that many of the numbers you may have seen reported in our blog posts for various OGD actions were reported for Fiscal Year 2023, which ran from October 1, 2022 through September 30, 2023. The Office of Generic Drugs 2023 Annual Report (here) is a bit different as it reports OGD actions from January 1, 2023 through December 31, 2023 (aka Calendar Year 2023); thus, there may be some data differences seen in various publications (including our blog posts) so be certain you are aware of whether the reported information is based on FY or calendar-year data.
With that in mind, the 2023 Annual Report from the OGD has a treasure trove of data as well as a laundry list of accomplishments that the program achieved in 2023. It also has a series of links in the appendix for conferences, public meetings, webinars, training programs, and workshops held over the year that you can access directly. There are also links to select online resources regarding the generic drug program and the review and approval process, along with a list of helpful acronyms and abbreviations for those of you that do not speak fluent FDA-ese.
If we look at the OGD from a by-the-numbers perspective for 2023, the report indicates that the OGD:
- Approved or tentatively approved a total of 956 ANDAs.
- Issued 1,493 complete response letters.
- Handled 101 pre-ANDA meeting requests.
- Had more than 21,000 stakeholders worldwide participate across eight public workshops and three webinars.
- Has published a total of 2,157 Product-Specific Guidances (PSGs) including the 158 new and revised PSGs published in 2023.
- Issued twelve policy documents that support generic drug development.
Here is a chart that looks at the 2023 numbers for approved and tentatively approved ANDAs. As you can see, there is quite a fluctuation month by month that makes predicting the trends a bit more challenging.
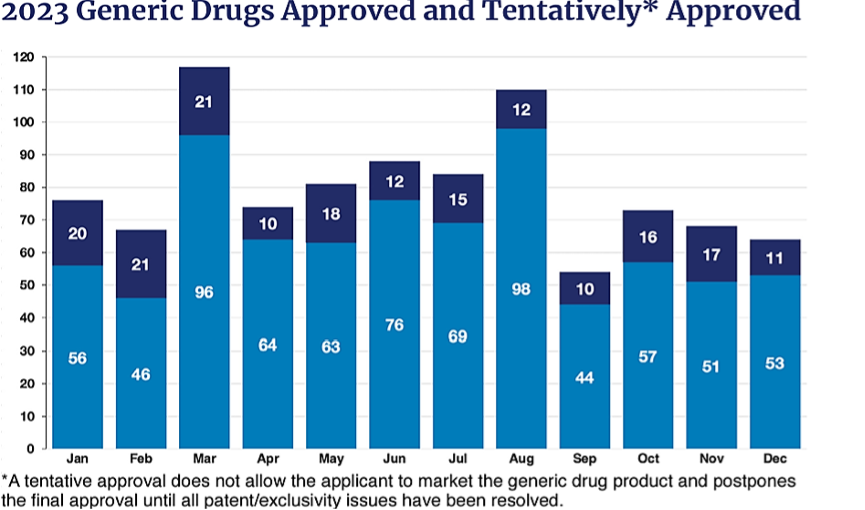
The report also indicates that the OGD approved ninety first-generic medicines in 2023 and lists several significant first ANDA approvals. In addition, the report discusses the Generic Drug Regulatory Science Research program and its components, as well as the impact that it has had on the review and approval process.
My advice is to keep the generic drug annual report bookmarked so you can easily retrieve the helpful information, especially the links that are provided. You can improve your knowledge of the inner workings of the OGD and the links provide an uncomplicated way to retrieve published guidances and other helpful information about the program.



