Just wanted to catch you up on a session related to generic drugs that I found particularly interesting presented by Edward “Ted” Sherwood, Director of Regulatory Operations, OGD, on some statistical metrics that are quite impressive.
Among other things, the presentation outlined missed GDUFA goal dates, but was not inclusive of those misses for imminent approval delays (applications delayed past goal date in an attempt to reach final approval or tentative approval in the current cycle).
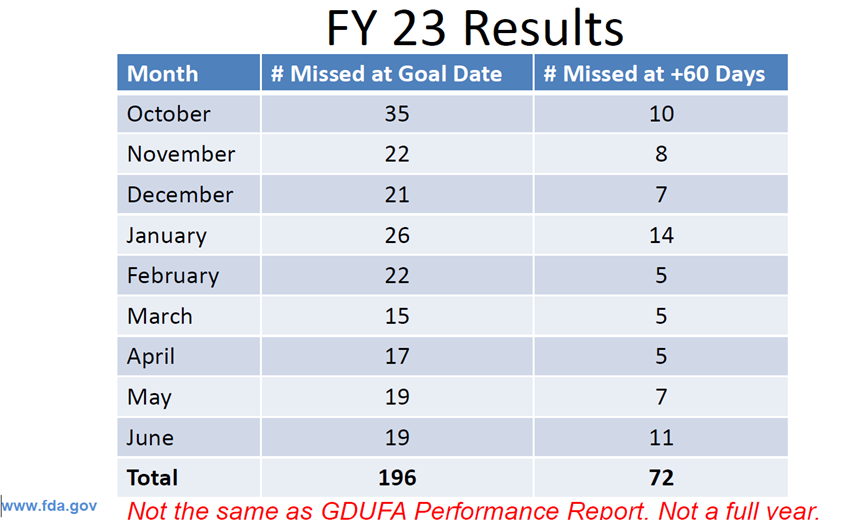
The slide above represents those ANDA that missed goal dates in FY 2023 however, this data was not for a full year. As a reference, at any time OGD has about 1550+ ANDAs pending. Action dates could be approvals, tentative approvals, or complete response letters.
But how do we know that GDUFA implementation has really made a difference for the first two full cycles of the program? The following two charts really tell the story. The first chart below shows approval times by cohort of receipt.
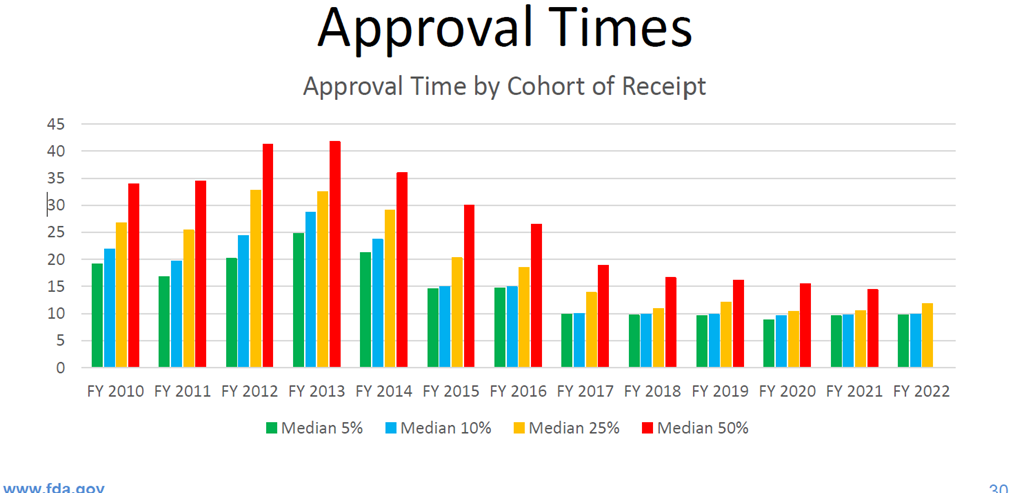
You can see the drops in mean and median approval times when looking at the cohort year, which mean newer ANDAs are getting approved faster but the older applications that are now being approved drag the mean and median numbers down in aggregate (when all approval times for all cohorts are combined).
The chart below displays a better comparison between GDUFA I and GDUFA II that clearly shows a steady cohort year decline in approval times. So, when you see the FDA quarterly mean and median approval time in some reports, remember they lump together applications approved that quarter for all cohort years.
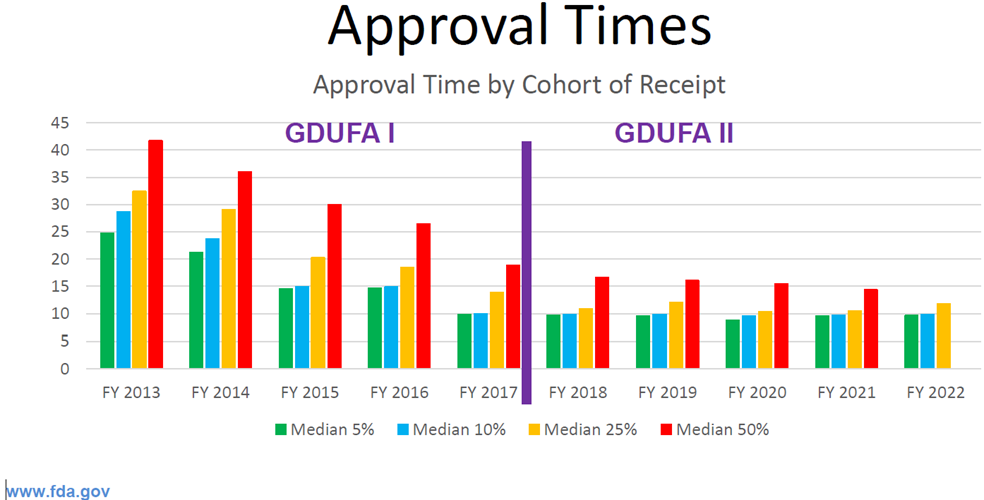
In speaking of missed goal dates, OGD stated that most were either due to imminent actions or thorny regulatory issues (e.g., nitrosamines). Clearly, with the thorny regulatory issues, there is no good way to predict when those issues may be resolved (and communication may be very fuzzy and not very forthcoming), but these issues receive a lot of post-goal date management oversight, according to the presentation. Communications for other misses are usually made to applicants to provide anticipated completion dates.
In addition, the presentation noted that prior approval supplements are being approved quite quickly with >97% being acted on time and most being approved well ahead of their assigned GDUFA goal date. That is some additional good news.
There will be more coming on the AAM’s GRx-Biosims conference in the coming days.



