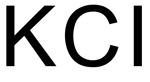On August 19, 2015, the FDA approved a second 505(b)(2) drug application for potassium chloride oral solution, a potassium replacement therapy typically for patients taking potassium-depleting diuretics. Previous to this approval, FDA approved another 505(b)(2) application for the drug on December 22, 2014.
Prior to that date, there were a number of marketed unapproved prescription drug products for potassium chloride oral solution on the market as “grandfathered” drugs or “DESI similar and related” drugs. The market for potassium replacement therapy is rather large and, while there are numerous approved solid oral dosage forms available in the marketplace, many patients cannot take those products for various reasons. Only time will tell, but one might think that this second potassium chloride oral solution approval may signal another enforcement action in the near future.