Acne Drug Recommended by Panel to Go Over the Counter
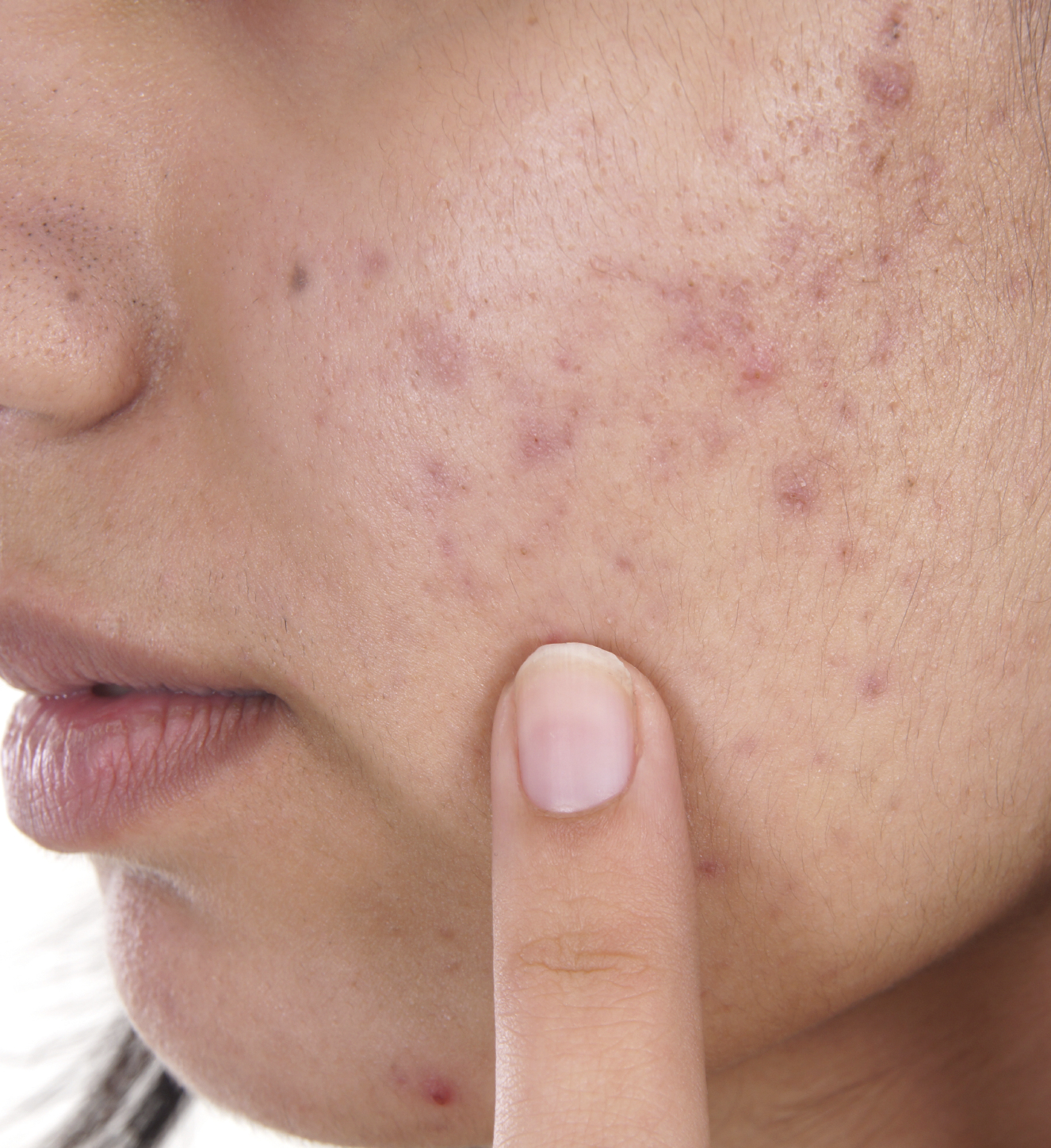
In an unusual 12-0 vote, an FDA advisory panel recommended that the acne treatment adapalene gel 0.1% be switched to Over-the-Counter (OTC) use. If the FDA concurs, this will be another FDA approved product to join a number of OTC monograph products in the OTC market to treat acne.